3.5 Label File
The Label File is optional and is used to customize labels of elements in the report—e.g., groups, column headers, etc. If the Label File is not specified, default labels will be applied to all report elements.
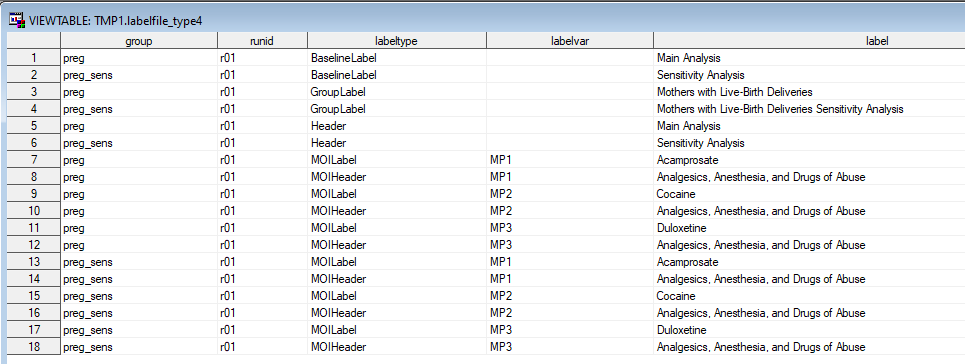
Figure 3.1: LABELFILE Example
| Parameter | Field Name | Description |
|---|---|---|
| GROUP/ANALYSISGRP/MILGRP to include in report | GROUP | Name of each cohort or analysis to include in report. |
| Note 1: GROUP must correspond to values of COHORTGRP/ANALYSISGRP/MILGRP included in distributed input files. | ||
| Note 2: for Type 6 (Manufacturer-Level Product Utilization and Switching Pattern) reports, include COHORTGRP values if requesting medical product utilization tables and/or ANALYSISGRP values if requesting switching tables. | ||
| Note 3: if a GroupLabel is not assigned for a group, the GROUP name will be displayed. | ||
| Note 4: if LABELTYPE is report-level, leave GROUP blank. | ||
| Format: SAS character $40; no special characters (e.g., commas, periods, hyphens, spaces, etc.) allowed, and underscores must be used to mark spaces. | ||
| Example: insulin | ||
| RunID Corresponding to GROUP | RUNID | RunID assigned to the run that corresponds to the group or analysis name listed in GROUP |
| Note 1: if LABELTYPE is report-level, leave RUNID blank. | ||
| Format: SAS character $5 | ||
| Example: r01 | ||
| Type of Label | LABELTYPE | Label to describe analyses in report titles. |
| Valid values are: | ||
Report-Level LABELTYPEs:
|
||
| Note 1: if >1 GROUP is included in 1 baseline table (using BASELINEGROUPNUM parameter in Baseline File), then the LABEL value for LABELTYPE = BaselineLabel should be the same for the two GROUPs appearing on the same table. Otherwise the program will default to LABEL value for the first GROUP listed. | ||
| Note 2: if LABELTYPE = AdherenceLabel, MOILabel, MOIHeader, CensorLabel, CFDDCatLabel, AFDDCatLabel, or CumDoseCatLabel, then LABELVAR must be specified. | ||
| Note 3: if no Header label is specified, all GROUPs will be displayed together with no header. If no MOIHeader label is specified, all medical products of interest will be displayed together with no header. | ||
| Note 4: if LABELTYPE is report-level, leave GROUP and RUNID blank. | ||
| Note 5: if ReportTitle is not defined, the default label “Exposures of Interest” will be used. | ||
| Format: SAS character $30 | ||
| Example: ReportTitle | ||
| Variable to Relabel | LABELVAR | Specifies the variable referred to by LABEL. |
| Valid values are: | ||
If LABELTYPE = CensorLabel:
|
||
If LABELTYPE = CFDDCatLabel, AFDDCatLabel, or CumDoseCatLabel:
|
||
If LABELTYPE = MOILabel or MOIHeader:
|
||
| Format: SAS character $32 | ||
| Example: cens_spec | ||
| Label to Apply | LABEL | Label (display name) for report element specified in LABELTYPE. |
| Format: SAS character - length can vary | ||
| Example: Beta Blockers |
3.5.1 Key Variables
The labelfile key variables are GROUP, RUNID, LABELTYPE, and LABELVAR. Users will who require multiple MOIHeaders will need to create two rows per MOI, one for LABELTYPE = MOILabel and one for LABELTYPE = MOIHeader. Table 3.8 illustrates this.| Labeltype | Labelvar | Label |
|---|---|---|
| moilabel | MP1 | Acamprosate |
| moiheader | Analgesics, Anesthesia, and Drugs of Abuse | |
| moilabel | MP2 | Cocaine |
| moiheader | Analgesics, Anesthesia, and Drugs of Abuse |